 |
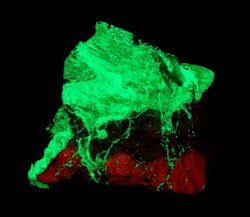
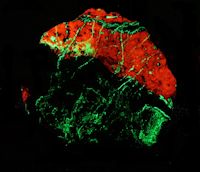
Collection Photo Gallery
|
 |
|
 |
Iíve found collecting at these two mines to be both a relaxing and rewarding experience.
|
 |
|
 |
 Mine Stock Certificates |
 |
|
 |
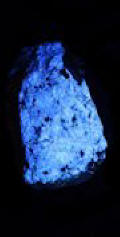
Collection Photo Gallery
|
 |
|
 |
Iíve found collecting at these two mines to be both a relaxing and rewarding experience.
|
 |
|
 |
 Mine Stock Certificates |
 |
|